Технология BioTrEM
Биоуправляемая целебная энергия - самая современная терапия заболеваний в домашних условиях!
Инновационные технологии управления здоровьем и долголетием - новые медицинские и бытовые аппараты КВЧ и фоновой резонансной терапии, основанной на технологии BioTrEМ
Продукция
- Аппарат «Триомед-Компакт»
- Аппарат «Триомед»
- Излучатели «Триомед»
- Кулоны здоровья BioTrem
- Продукция РаВита (RaVita)
- Аппарат Триомед Корректор (Corrector)
- Аппарат Триомед Энергия жизни (Life Energy)
- ЭННИ RaVita (РаВита)
- ЭННИ Optima (Оптима)
- Гармонизатор «CGI»
- Дыхательный тренажёр
Как заказать
Контакты
Консультационный центр
Методология
- Концепция управления здоровьем
- Научно-исследовательская работа
- КВЧ-терапия в медицине
- ФРИ-терапия
- Переводы на ENG
- Диссертации по КВЧ
- Утверждено Минздравом
- Авторские методики
- Презентация продукции
- О разработчиках
- Клинические испытания
- Характеристики
- Инструкции
- Область применения
- Отзывы
- Литература-каталог
- Видео-каталог
- Видео-презентации и мероприятия
- Видео-авторские наработки
- Видео-лекции по заболеваниям
Статьи
Вопрос/ответ
Фото
Атлас БАТ
Новости
Instrument for EHF-infrared therapy "Triomed". Operational manual
APPROVED
By Resolution of Roszdravnadzor
Of December 28, 2009 № 10684-Pr/09
INSTRUMENT FOR EHF-INFRARED THERAPY "TRIOMED"
ТU 9444-014-61005106-2009
Operational manual
ТGКB 941.526.001IP
Saint-Petersburg, 2010
Application manual was developed by OOO Triomed.
The document is not subject to copying or transferring to other organizations and persons without consent of the owner.
1. PURPOSE
EHF-IR therapy “Triomed” device is a reflexological, physiotheraputical and medical instrument for the treatment and prevention of various pathological conditions by exposure to low-intensity electromagnetic radiation of EHF and infrared ranges on human skin.The inclusion of EHF therapy in complex treatment of many diseases allows to reduce drug dosage, potentiate drug action, to avoid using drugs in some cases, improve toleration of many drugs, reduce severity of side effects, achieve positive clinical results for drug-resistant patients.
The device can be used by general medical, medical and preventive institutions as well as by an individual under the supervision of a physician in a hospital, outpatient and at home.
As a result of experiments, clinical trials and medical practice, the following effects of EHF-infrared therapy were noticed and studied in depth:
• improved sensitivity of receptors of the membrane and the cell nucleus;
• normalized activity of central nervous system;
• stimulated intracellular synthesis of cyclic nucleotides cAMP, cGMP;
• modulated activity of various parts of the immune system;
• activated functioning of the diffuse neuroendocrine system;
• activated lipid peroxidation - antioxidant protection;
• improved permeability of blood capillaries;
• improved rheological blood properties and restoration of blood homeostasis;
• optimized hormonal status;
• radioprotective effect.
These effects are clinically proved to have anti-inflammatory, analgesic and anti-edematous results; improved processes of tissue regeneration, enhanced non-specific resistance of the organism, improved systemic and regional hemodynamic; antistress effect, normalized autonomic nervous system as well as several other clinical and physiological results.
2. MAIN CHARACTERISTICS
Main characteristics of the device are specified in the operational manual.3. INDICATIONS FOR USE
• diseases of peripheral nervous system;
• diseases of autonomic nervous system;
• drug abuse treatment;
• otolaryngological diseases;
• diseases of cardiovascular system;
• diseases of lungs and pleura;
• diseases of the digestive tract;
• diseases of skin and subcutaneous tissue;
• diseases of musculoskeletal system;
• gynecological diseases;
• pain relieve;
• joint pathology;
• spine diseases;
• wound and burn treatment;
• allergic skin reactions.
4. CONTRAINDICATIONS FOR USE
• general contraindications for physiotherapy;
• uncertain diagnosis;
• individual allergic reactions on the use of the device
• fevers of unknown etiology
• If a patient has implants with autonomous power supply (in the area of the device installation).
5. THE UNIT COMPONENTS, DESIGN AND PERFORMANCE
5.1. Components5.1.1. The unit consists of:
• low-frequency electronic generator "Triomed" TU 6349-005-61005106-2009 (hereinafter "power supply")
• remote transmitters (from number 1 to number 5) TGKB 941.526.001-005,
• standard cable USB А – mini USB 5 pin,
• two batteries LR06 (AA) with a nominal DC voltage 3.0 V.
5.1.2. Power source, an electronic circuit for a pulsed power semiconductor transmitters and the control unit are located in the plastic case of the power supply. Voltage is supplied from the control unit to the transmitter. Device transmitter (semiconductor generator and antenna) are located in a plastic case.
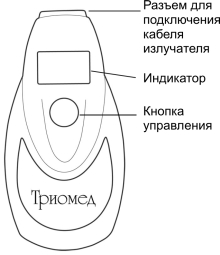
5.1.3. The following elements are located on the front panel of power supply:
• Three-character 7-segment LED,
• Device control button.
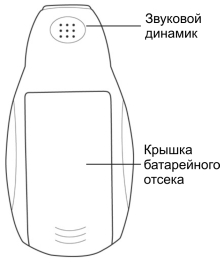
5.1.4. The following elements are located on the rear panel of the power supply:
• The battery compartment cover
• Speaker, sounding reminiscent of chatter during radiation.
5.1.5. In the upper part of the power supply, a standard USB A-1 connector is located for connecting a standard USB cable A with mini USB 5 pin, connected to the transmitter.
|
|
|
Figure. 1. Frontal view.
General view of the device front panel |
Figure. 2. Rare view |
5.1.6. Each radiator has an LED installed (it signals on the transmitter’s performance), connector and mini USB 5 pin cable connector.
5.1.7. The device is switched on automatically after the transmitter is connected, at that the according mode is automatically set while the indicator displays the power supply number.
5.1.8. After pressing the control button, radiation starts generating, the indicator displays the time period (in seconds) during which the device operates in a set mode; the countdown time starts immediately.
5.1.9. The radiation may be switched off by double pressing the control button.
5.1.10. The device shuts down automatically within 10 seconds after the set time of the selected mode elapses or in if the button is not pressed.
5.2. Configuration.
The device may be delivered in different configuration upon customer’s request. Configuration is stated on the label on the device.
6. PREPARATION OF THE DEVICE BEFORE THE START OF OPERATION
6.1. Operational limitations6.1.1. Using the device is allowed only after reading the Operational Manual and these Regulations.
6.1.2. It is prohibited:
• To stare at the IR transmitter: on the radiating (unmarked) surface at a distance of less than 50 cm from the eye;
• To keep the instrument in places accessible to children and pets, give transmitters to children;
• to use saline batteries marked R06 as a power source. These batteries have a low shelf life, after the end of which they disintegrate, polluting and damaging the environment. It is advisable to use batteries marked LR06 («ENERGIZER», «GP», «PANASONIC»);
• to use a self-made power source;
• Connect the device and transmitters via USB and mini USB to other equipment;
• to subject the device and transmitters to excessive mechanical impacts, falls.
• To bend and break connecting cable, stretch it when cleaning, pull the cable when turning off the transmitter;
• To place the device on operating home appliances.
6.1.3. Safety measures. In case of device operational failure, emergency conditions or emergency evacuation of medical personnel, no special security measures are required.
6.1.4. It is recommended to wear cotton clothes before using the device.
6.1.5. It is recommended to conduct treatment procedure with a patient sitting or laying down.
6.1.6. Do not spill the water and chemicals inside the device (transmitter) and on its case.
6.1.7. The outer surface of the device is cleaned and disinfected according to MU 287-113 with a 3% hydrogen peroxide solution with the addition of 0,5% solution of detergent (washing powder) with soaked and wrung out cloth.
6.1.8. To start using the device and transmitters after storage at temperatures below 0°C, one can no earlier than 4 (four) hours after it has been exposed to a room temperature while unpacked.
6.1.9. When transporting the device, it is easy to use consumer packaging. To ensure maximum safety pack the unit once again like it was originally packed at the factory.
6.2. Preparation of the device before use
6.2.1. Before turning on the device, conduct an external inspection of the device and make sure there is no damage of the case. Operation of the device with a damaged case is FORBIDDEN!
6.2.2. Functional test:
Connect the transmitter to the device. The unit turns on; the indicator displays the number of selected mode;
• Click control button. Radiation starts emitting accompanied with a crick-cracking sound from the speaker; the indicator displays time (in seconds) of the device operating in the mode of the connected transmitter, the countdown time begins. The LED on the transmitter switches on;
• Click control button again, do not wait untill the countdown time goes to zero. All indicator decimals display zeros, crick-cracking sound stops; LED on the indicator switches off. In 10 seconds the indicator panel switches off, the device is switched off.
6.2.3. Replacing batteries.
Discharge of batteries is determined based on the brightness of the indicator segments. Lack of light and sound signals the device malfunction or a battery discharge. You should check the battery and, if necessary, replace it. If this happens due to the unit malfunction, go to the address listed in your passport for inspection and repair.
To replace the batteries, one needs to open the battery compartment cover (see Fig. 2) at the rear side of the device, remove old batteries and insert new ones, complying with polarity, in accordance with the marking on the device and on the batteries.
6.3. The list of possible defects and its elimination.
Possible defects and its elimination are specified in Table 3.
| № | Defect symptoms | Possible cause | Elimination |
| 1 | When connecting transmitter, power supply indicator does not display its number | Batteries malfunctioning or discharged | Replace batteries. If replacing with good batteries did not help, send device for repair. |
| 2 | Same | No contact in connectors on the device or in the transmitter. | Reinstate contact in connectors (redock transmitter connector). |
| 3 | Same | Transmitter malfunction | Identify faulty transmitter by connecting other transmitters consequently, send it in for repair |
| 4 | Same | Transmitter connecting cable malfunction | Replace the cable with a new one |
| 5 | After pressing control button, crick-cracking sound does not come on, indicator does not display time, countdown does not begin, LED on the transmitter is not switched on | Device malfunction | Send the device in for repair |
In case of other faults, contact the manufacturer or its official representative. Addresses and contact numbers are listed in the passport.
6.4. Technical maintenance
Technical maintenance of the device (test performance and characteristics of EHF and infrared radiation) is conducted once a year at repair and warranty service offices.
7. DEVICE OPEARTION
7.1. Clinical application of the device is governed by these Regulations.7.2. The points of exposure when using "Triomed" device in accordance with rules and principles of physical therapy, reflexology, and regenerative medicine could be: the projection area of biologically active points, the biologically active zones, the pathological focus or an area of its projection, direct projection of organs, spine area, joints and major vessels, the projection of organs in the Zakharyin-Ged areas (Fig. 3). Point and zonal approaches may be executed during one procedure. When using the device, one may use methodological recommendations, new and advanced medical technologies, doctors manuals, approved by Roszdravnadzor (Appendix 1).
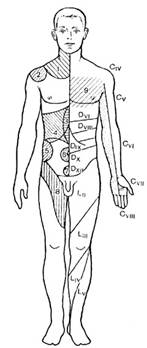 Figure 3. Projection zones of Zakharyin-Ged
Figure 3. Projection zones of Zakharyin-Ged
(1 – lungs, 2 – liver; 3 – stomach and pancreas; 4 – liver; 5 – kidney; 6 – small intestine; 7 – colon; 8 – urethra; 9 – heart)
7.3. Depending on location of the pathological focus, degree of clinical syndromes, disease stage and the initial state of the organism, treatment must be selected individually: location and duration of exposure, type of transmitter, and number of medical procedures.
7.4. Time of exposure on one area or biologically active point is from 3 to 15 minutes. The total time of exposure should not exceed 30 minutes per day.
7.5. During the initial period of treatment (1-2 days), it is advisable to use the device to gradual activate regulatory systems (1 treatment every 1-2 days). After adaptation, the intensity of treatment increases to 2-3 procedures par day. If necessary, treatment can be repeated after 2-3 months.
7.6. Recommendations for the use of transmitters, depending on the disease:
• transmitter with red label (from 7,5 to 6,98 mm) - vitiligo, herpes, psoriasis, cholecystitis, periodontitis and other mouth diseases, blood formation, vegetative-vascular dystonia;
• transmitter with green label (from 5,77 to 5,26 mm) - neuro-dermabrasion, eczema, post-traumatic contractures, asthma, sarcoidosis, appendagitis, vegetative-vascular dystonia;
• transmitter with blue label (from 5,26 to 4,76 mm) - obstructive bronchitis, peptic ulcer of stomach and duodenum, immunostimulation;
• transmitter with yellow label (from 6,0 to 4,0 mm) - for children from 3 to 6 years old, debilitated patients as preparation for main medical treatment, to optimize the total (nonspecific) resistance .
• transmitter with white label (infrared radiation) - chronic and subacute inflammatory disease process, stimulation of reparative regeneration processes on final stages of inflammatory process, analgetic effect, improving micro-circulation and permeability of capillary walls.
7.7. Recommended number of treatments is from 7 to 15, the frequency of 1-3 times per day depending on a patient's condition.
7.8. To ensure continuity of outpatient treatment, it is recommended to use the device at home under medical supervision. To increase the effectiveness of treatment at home, it is recommended to use the projection of organs in the areas Zakharyin-Geda along with the projection area of biologically active points for greater therapeutic effect.
7.9. Attention: If you experience unpleasant sensations, which do not disappear after 3 procedures, your condition worsens, please consult your doctor.
7.10. Procedure description
• A patient takes comfortable position.
• Before starting the treatment procedure, select the transmitter and connect it to the device via a cable. The display shows the mode number.
• Set the transmitter on the patient's body color label facing up and fix it, holding with your hand or with a tape. When working on biologically active zones, it is recommended to move the transducer slowly using circular motions, on the spinal column, major vessels and main vessels - use longitudinal motions.
• Turn on the device by pressing control button. The display shows duration of the session, the LED on the transmitter is lit, speaker makes the sound.
• Wait till the procedure is over. When the procedure ends, the device turns off automatically.
• To abort the procedure, press control button.
8. STORAGE RULES
8.1. StorageConditions of storage of the device in the manufacturer’s packaging in warehouses of either a manufacturer or a customer must meet the conditions of storage 2 according to GOST 15150.
8.2. Manufacturer warranty
Manufacturer warranty is specified in the operational manual.
8.3. Recycling
Device shall be disposed of in the container specially designed for radio-electronic equipment.
ANNEX 1
Medical recommendations and technology approved by Roszdravnadzor RF- EHF-therapy in preparatory phase of surgical treatment of ischemic heart disease (EHF-therapy).
Methodical recommendations № 99/193 (approved by the Ministry of Health on 25.05.2001).
Authors: PhD, Senior Scientist O.E Golosov, MD, Professor E.F Levitsky, MD, Professor T. D. Gridnev, MD A. Cherniavsky, PhD. A. Kozhemjakin. - Integrated physio-balneotherapy of chronical prostatitis
(integrated treatment with EHF therapy).
Improved medical technology (registration certificate number FS-2006/023-u of March 11, 2006, issued by the Federal Service for Supervision of Health and Social Development).
MD, Professor E.F Levitsky, Senior Researcher, Ph.D. I,A Kolmatsuy, researcher, PhD E.A Neplokhov, Ph.D. A. Matveev, MD O.E Golosov. - Chronobiological approach to rehabilitional treatment of patients with osteoarthrosis in siberian region
(integrated treatment with EHF therapy).
New medical technology (registration certificate Number FS-2006/047 of April 10, 2006 issued by the Federal Service for Supervision of Health and Social Development).
Authors of technologies: Honored Scientist of RF, MD, Professor E.F Levitsky, phD E.V Titskaya, MD G.G Reshetova, MD N.G Abdulkina, MD E.V Mikhailova, MD D.I Kuzmenko. - Optimization rehabilitation treatment of patients with osteoarthrosis in combination with flatfoot
(integrated treatment with EHF therapy).
Improved medical technology (registration certificate number FS-2006/126-u of 14 June 2006. Issued by the Federal Service for Supervision of Health and Social Development).
Authors: MD E.V Titskaya, MD G.G Rechetov, MD E.V Mikhailov, MD N.G Abdulkina, MD O.V Dostovalova, Ph.D. V.F. Savrasov, MD O.M. Tkachenko, MD V.D. Zavadovskaya, MD T.B. Perova - Chronobiological optimization physio-balneotherapy hypertensive patients in siberian region
(integrated treatment with EHF therapy).
New medical technology (registration certificate Number
FS-2006/078 of 10 May, 2006. Issued by the Federal Service for Supervision of Health and Social Development).
Authors: MD, Professor E.F. Levitsky, MD I.N. Smirnova, MD N.G. Abdulkina, doctor L.M.Nikonov, a doctor L.S. Yakushev, MD O.E. Golosova, MD V.V. Bezlyak, MD S.V. Alaytseva, doctor I.Y. Lyapunov, MD S.S. Shakova, a physician N.V. Merzlyakova, researcher E.A. Tyumentseva. - Electromagnetic radiation of millimeter range in rehabilitation of patients with ischemic heart disease
(integrated treatment with EHF therapy).
New medical technology № 98/31 (approved by Ministry of health 18.06. 1998 ).
Authors: MD, Professor E.F. Levitsky, MD I.N. Smirnova, MD N.G. Abdulkina, doctor L.M.Nikonov, a doctor L.S. Yakushev, MD O.E. Golosova, MD V.V. Bezlyak, MD S.V. Alaytseva, doctor I.Y. Lyapunov, MD S.S. Shakova, a physician N.V. Merzlyakova, researcher E.A. Tyumentseva. - EHF-therapy for patients with vertebrogenic neyrodystrophic pseudo cardialgia (syndrome of secondary scalinus)
(EHF-therapy).
Methodical recommendations № 2002/74 (approved by the Ministry of Health on 28/02/2003).
Authors: MD, Professor E.F. Levitsky, MD L.P. Strelis, MD O.E. Golosova, Ph.D. O.N. Markov. - Millimeter waves in the treatment of patients with neurological manifestations of spinal osteochondrosis
(EHF-therapy).
New medical technology № 2000/199 (approved by the Ministry of Health in 2000)
Authors of the technology: MD, professor, Honored Scientist of RF, E.F. Levitsky, MD N.F. Miryutova, Research Assistant I.M. Mavlyautdinova, A.M. Kozhemjakin. - Electromagnetic radiation of ehf-range in recovery treatment of neuro-orthopedic disorders in patients with lumbar osteochondrosis, children and adolescents with idiopathic scoliosis
(Integrated treatment with EHF-therapy).
Improved medical technology (registration certificate number FS-2006/039-u of April 10, 2006, issued by the Federal Service for Supervision of Health and Social Development, valid until November 11, 2009).
Authors of the technology: MD, professor, Honored Scientist of RF, E.F. Levitsky, MD, Professor N.F. Miryutova, MD N.G. Abdulkina, Ph.D. A.M. Kozhemyakin, E.V. Lipina, N.N. Bartfeld. - Recovery treatment of patients with lyme borreliosis physiotherapeutic factors
(Integrated treatment with EHF-therapy).
Methodical recommendations № 2002/75 (approved by the Ministry of Health on 28.02.2003, valid until 28.02.2013).
Authors: MD G.G. Reshetova, MD A..A Zaitsev, MD E.V. Titskaya, PhD N.G. Abdulkina, PhD, Professor V.D. Zavadovskaya, MD T.B. Perova, Physician I.M. Mavlyautdinova, Physician O.V. Dostovalova. - Using resonant radiation as a background treatment for relief of pain syndromes in neyrovertebragenous diseases
Medical advice № 99/91 (approved by the Ministry of Health of 29.11.1999).
Authors: PhD I.L. Blinov, PhD L.E. Gedymin, PhD E..F Levitsky, PhD VI Mikhailov, Ph.D. A.M. Kozhemjakin, MD, I.N. Brant, N.N. Dmitriev, O.G. Golosova. - Integrated application of methods of physio-balneotherapy in treatment of hysteromyoma
(Integrated treatment with EHF-therapy)
Methodological manual 1998. (approved by the Ministry of Health in 1998).
Authors: PhD I.I. Diamant, MD, Ph.D. G.B. Dikke, Ph.D. Y.F. Ruzaeva. - Application of ehf-therapy in complex treatment of various forms of psoriasis
(Integrated treatment with EHF-therapy).
Methodological recommendations (approved by the Ministry of Health in 2001).
Authors: PhD V..S Dmytruk, PhD O.E. Golosova, MD E.F. Levitsky. - Treatment of females after surgery on the uterus and its appendages using EHF-therapy
Methodological recommendations (approved by the Ministry of Health in 1998).
Authors: PhD I.I. Diamant, MD, Ph.D. G.B. Dikke, Ph.D. Y.F. Ruzaeva, Dobkina. - EHF-therapy in treatment of patients with hysteromyoma combined with fibrocystic breast disease
Methodological recommendations (approved by the Ministry of Health in 2001).
Authors: PhD G.B. Dikke, MD, Professor. T.D. Gridneva, MD, Professor. S.A. Velichko. - Restoration of sexual and reproductive health of men, suffering from excretory-toxic infertility on a sanatorium stage of treatment
Methodological manual for doctors (approved by the Ministry of Health in 1998).
Authors: PhD, senior scientist I.A. Kolmatsuy, PhD, Research Scientist E.A. Neplokhov, a doctor O.K. Vysotina. - Integrated physio-balneotherapy of climacteric disorders of men
Methodological manual for doctors (approved by the Ministry of Health RF in 2001).
Authors: PhD, senior scientist I.A. Kolmatsuy, Ph.D., senior scientist E.A. Neplokhov. - EHF-therapy of post-resection disorders in patients radically operated for stomach cancer on sanatorium recovery phase
Methodological recommendations №98/12 (approved by the Ministry of Health RF in 1998).
Authors: PhD T.Y. Kucherova, MD, Professor. E.F. Levitsky, MD B.N. Zyrianov, MD S.V .Nizkodubova, MD E.I. Beloborodova, PhD Yevtushenko A.A. - EHF-therapy in complex treatment of atopic dermatitis in children
Methodological manual for doctors (approved by the Ministry of Health RF in 2001).
Authors: MD prof. T.D. Gridneva, MD, Professor. P.N. Pesterev, PhD O.E. Golosova, doctor E.V. Perminova, under the general editorship of MD, Professor E.F. Levitsky.